[ad_1]

No Covid infection has been reported among children who received the two doses of the vaccine.
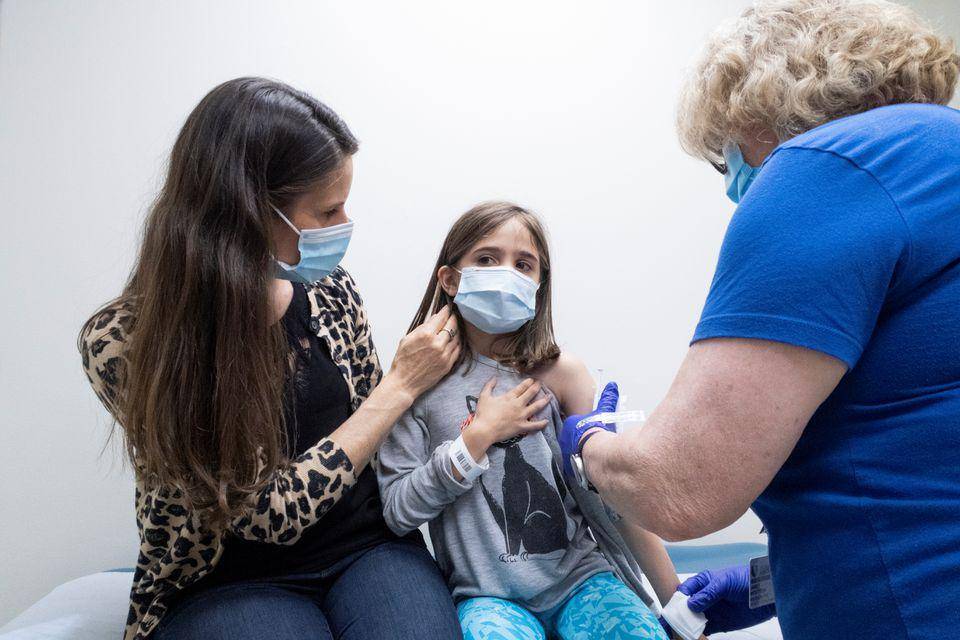
Among the children aged 3 to 17 who participated in the Sinopharm immune bridge study, more than 96% developed antibodies against Covid-19.
The study shows that the vaccine is very effective in preventing Covid-19 because none of the children who have received the two injections have been infected with the coronavirus.
More than 900 children participated in the research conducted in Abu Dhabi.
Earlier this month, the UAE authorities approved the Sinopharm vaccine for use in children of this age.
Officials did not observe any serious side effects in the children participating in the study. Among the vaccinated children, 29.7% felt pain at the injection site; 8% had headaches and 3.7% had fever.
The children overcome the minor side effects within a few days.
The preliminary results of this research will help support the planning process for students to return to school safely.
The study was carried out under the supervision of the Ministry of Health and Prevention (MoHAP), with full parental consent. All young volunteers are closely monitored and taken care of at every step of the process.
The authorities advise parents to vaccinate their children.
The new crown vaccine approved for children aged 3 to 11 is Sinopharm; and those between 12 and 15 years old can be given to Sinopharm or Pfizer Biotechnology.
The UAE is the first country in the Middle East and North Africa to conduct a vaccine effectiveness study for this age group. Other vaccine-producing countries, such as China, the United States, the United Kingdom, and India, have conducted similar clinical trials in the past few months.
sahim@khaleejtimes.com
Sahem Salem
[ad_2]
Source link