[ad_1]

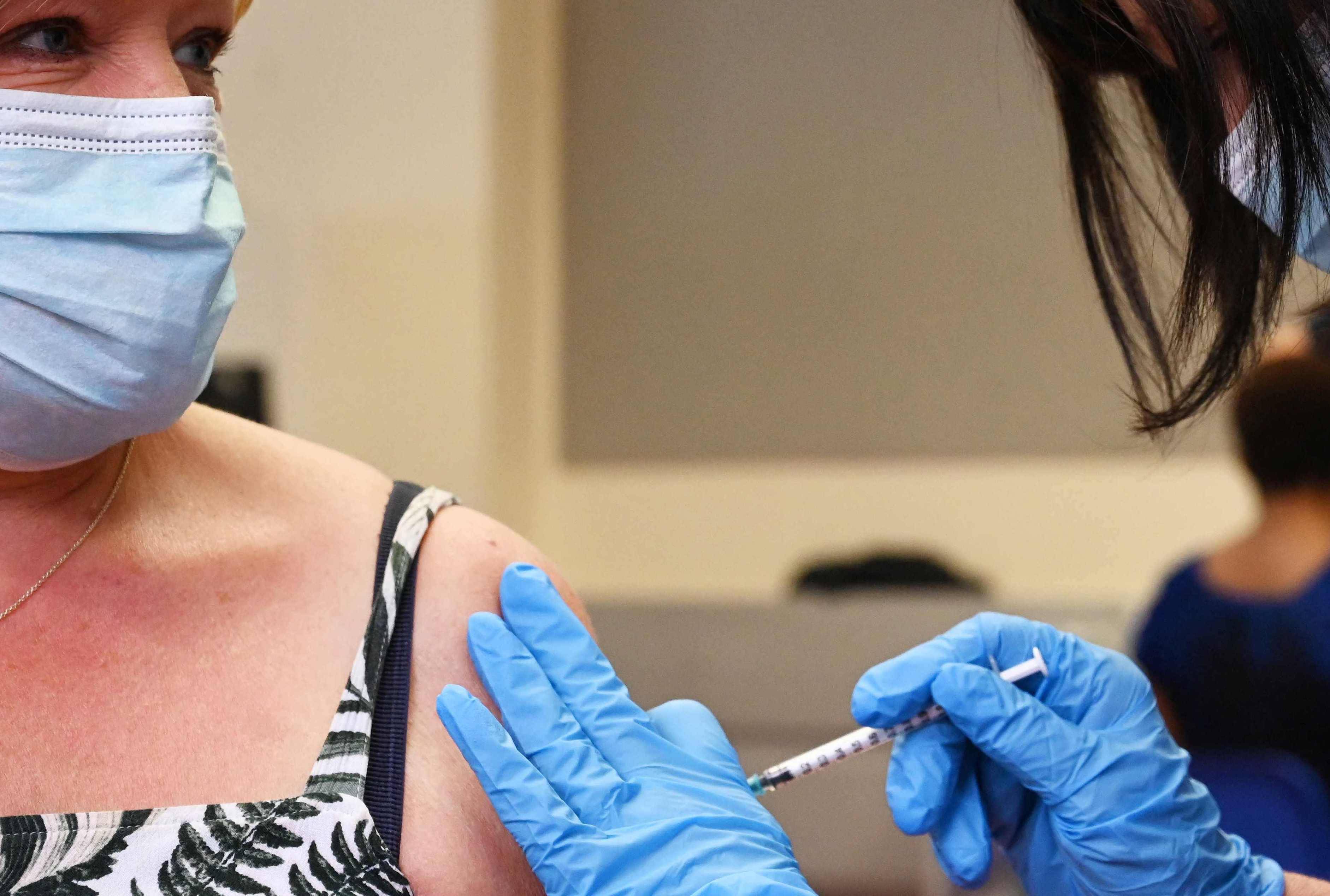
According to the authorization, Americans who have been vaccinated are eligible to receive the third dose 6 months after receiving the second Pfizer injection.
With the US Food and Drug Administration’s approval of targeted use of additional vaccines, the United States on Wednesday is closer to providing a booster dose of Pfizer’s Covid-19 vaccine to the elderly and other people at high risk of contracting the virus.
The FDA authorizes booster doses for Americans 65 years and older, young people with underlying health conditions, and those engaged in high-risk Covid-19 work. The ruling represents a greatly reduced version of the Biden administration’s comprehensive plan to provide almost all American adults with a third dose of vaccine to strengthen their protection during the spread of the highly contagious delta virus.
However, there are more regulatory hurdles before starting to distribute boosters.
Consultants from the Centers for Disease Control and Prevention held a two-day meeting on Wednesday to give their own more specific recommendations on who should receive the additional injections and when. On the first day of the discussion, some experts were very confused about the reasons surrounding the booster, and they suggested that the decision should be postponed for a month in order to obtain more evidence.
The uncertainty again reminds people that the science surrounding boosters is more complicated than the Biden administration implied when the President and his senior aides unveiled their plans at the White House last month.
After the FDA’s own advisory panel rejected Biden’s plan by an overwhelming majority last week, the FDA is expected to make a decision on Wednesday. Instead, the team recommends using boosters only for those who are most vulnerable to severe cases of Covid-19.
FDA Acting Commissioner Dr. Janet Woodcock said in a statement that the FDA’s authorization will allow boosters for medical staff, teachers, grocery store workers, and people in homeless shelters or prisons.
“As we learn more about the safety and effectiveness of the Covid-19 vaccine, including the use of enhanced doses, we will continue to evaluate the rapidly changing science and keep the public informed,” Woodcock said.
According to FDA authorization, Americans who have received the vaccine are eligible for the third dose six months after receiving the second Pfizer injection. This is different from the proposal Biden announced in August, which called for support eight months later.
White House Press Secretary Jen Psaki said on Twitter Wednesday night: “Today’s FDA decision is an important step in our efforts to provide Americans with additional Covid-19 protection.” Give a booster shot and plan to do so later this week in accordance with the final recommendation of the CDC.”
Given that the agency usually takes action before the CDC convenes its own experts, the timing of the FDA’s decision is very unusual.
CDC team members listened to a series of presentations on Wednesday, outlining the tricky state of booster science. On the one hand, the Covid-19 vaccine continues to provide strong protection against serious illness, hospitalization and death. On the other hand, as immunity declines, there are more signs of low-grade infections among vaccinated people.
Ultimately, the committee must decide who is deemed to be at a sufficiently high risk to require additional doses. Data provided by Pfizer and the Israeli government indicate that people aged 65 years and older need to intensify injections, but there is no evidence that additional injections are of great benefit to young people with underlying health conditions.
Several CDC consultants agreed that boosters are also important to keep medical staff working.
“We don’t have enough medical staff to take care of people who have not been vaccinated,” said Dr. Helen Keipp Talbot of Vanderbilt University. “They just keep coming.”
The Centers for Disease Control and Prevention has stated that it is considering providing boosters for the elderly, nursing home residents and front-line medical staff, rather than all adults.
The World Health Organization and other global health advocates oppose the third round of injections in rich countries when poor countries do not have enough vaccines for the first dose. Many independent scientists stated that these vaccines continue to perform well against the worst effects of Covid-19, and their ability to contain the overall trajectory of the epidemic is uncertain.
US regulators will decide later whether to provide boosters for people vaccinated by Moderna or Johnson & Johnson. They stated that they do not recommend Pfizer vaccines to people who were initially vaccinated with different brands of vaccines.
The full rollout of the booster proposed by the White House should have started this week. Some people questioned whether President Joe Biden announced his plan before government regulators came to any conclusions, thus being at the forefront of science.
Despite resistance in recent days, some senior US health officials said they expect the booster will eventually gain wider approval in the coming weeks or months. Dr. Anthony Fauci said over the weekend, “This is not the end of the story.”
Other government officials pointed out that the FDA’s decision covers tens of millions of Americans, and even if additional injections have been approved for the entire population, the elderly and other high-risk groups will be the first to be vaccinated. In December of last year, seniors were the first Americans eligible for vaccination.
The United States has approved the third dose of Pfizer and Moderna vaccines for certain people with weakened immune systems (such as cancer patients and transplant recipients). Other Americans, whether healthy or not, have managed to obtain boosters, in some cases only through questioning.
The United States receives an average of about 760,000 vaccines per day, which is lower than the high of 3.4 million vaccines per day in mid-April. Approximately 180 million Americans have been fully vaccinated, accounting for 64% of eligible people.
[ad_2]
Source link