[ad_1]

After regulatory approval, shooting may begin early next month.
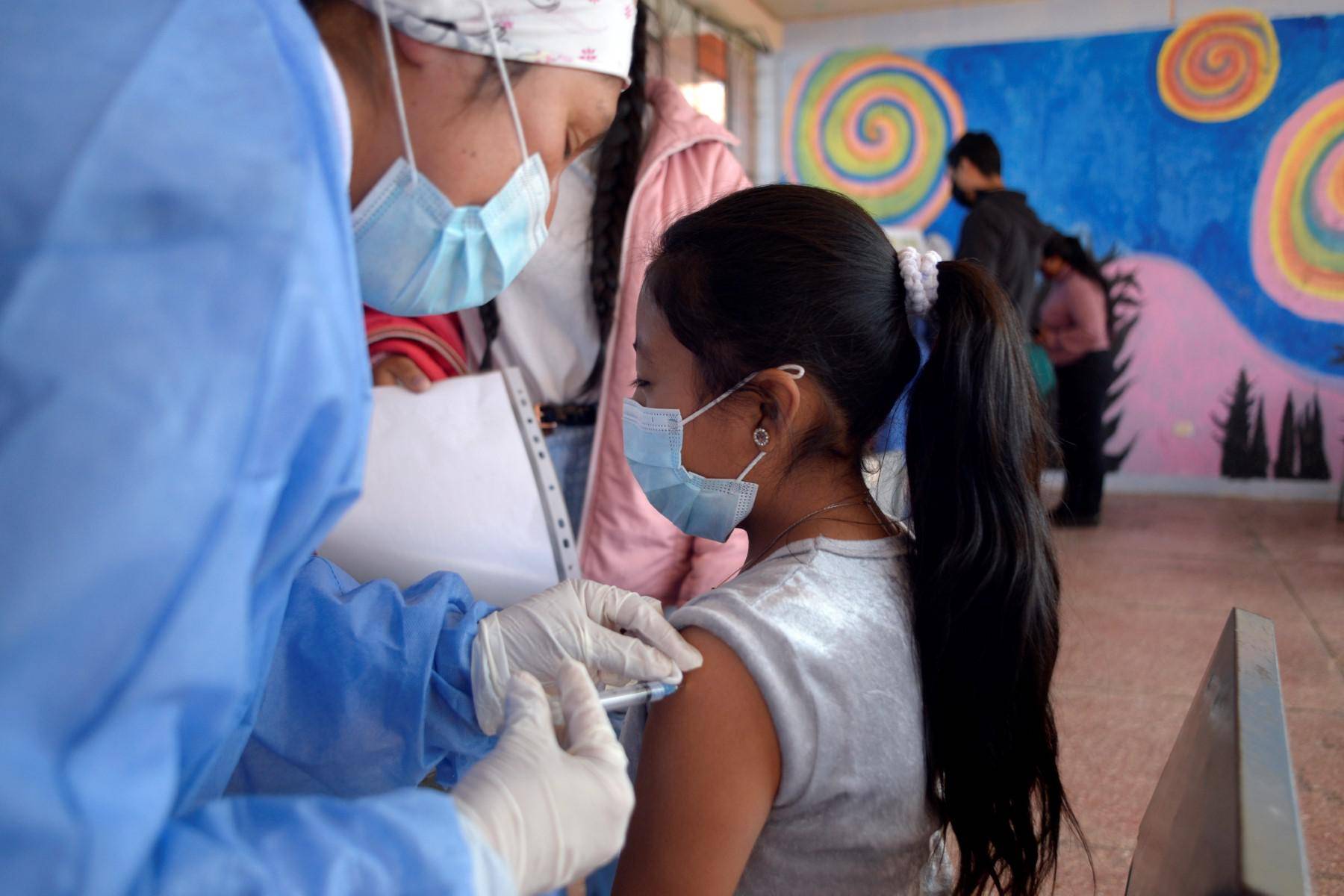
Pfizer stated that the child dose of its Covid-19 vaccine is safe, and its effectiveness in preventing infection among elementary school students is close to 91%.
According to research details released on Friday, it can protect children aged 5 to 11 years from symptomatic infections because the United States is considering opening up vaccination for this age group.
Please also read:
>> The Indian economy accelerates with the increase in Covid vaccination coverage: PM Modi
>> With more than 115,000 deaths, WHO urges vaccinations for health workers
If approved by regulatory agencies, these vaccines may start early next month-the first children in line will be fully protected by Christmas.
Details of the Pfizer research will be published online on Friday. Advisors from the U.S. Food and Drug Administration will openly debate the evidence next week.
The FDA is expected to release an independent review of the company’s safety and effectiveness data later on Friday. If the FDA finally approves the injection, the Centers for Disease Control and Prevention will make a final decision on who should receive the injection in early November.
Pfizer full-dose injections have been authorized for anyone 12 years of age or older, but pediatricians and many parents are anxiously waiting for their young children to be protected to prevent the increasing infection caused by the highly contagious delta variant. And help the children to continue to school.
More than 25,000 pediatricians and primary care providers have signed up plans to use these vaccines in small arms.
The Biden administration has purchased enough child doses for approximately 28 million children between the ages of 5 and 11 across the country-packed in special orange bottle caps to distinguish them from adult vaccines. If the vaccine is eliminated, millions of doses of vaccine will be quickly shipped across the country along with child-sized needles.
A Pfizer study tracked 2,268 children in this age group who received two placebo or low-dose vaccines in three weeks. Each dose is one-third of the dose given to adolescents and adults.
The researchers calculated that the effectiveness of the low-dose vaccine was close to 91%, based on 16 cases of Covid-19 vaccinated adolescents and 3 cases of vaccinated children. No young people have reported serious illnesses, but the symptoms of vaccinated people are much milder than their unvaccinated counterparts.
In addition, young children who received low-dose injections produced as strong anti-coronavirus antibody levels as teenagers and young people who received regular-intensity vaccinations.
This is important information considering that the majority of unvaccinated children hospitalized last month reached record levels.
The CDC reported earlier this week that even with a surge in delta mutants between June and September, Pfizer vaccination is 93% effective in preventing hospitalization of children 12 to 18 years old.
Pfizer’s research on young children found that low-dose injections proved to be safe, with temporary side effects similar to or less than those of teenagers, such as arm soreness, fever, or pain.
The study is not sufficient to detect any extremely rare side effects, such as the occasional heart inflammation after the second dose, which mainly occurs in young men.
According to the CDC, although the risk of serious illness or death in children is lower than that of the elderly, Covid-19 has killed more than 630 Americans 18 and younger. According to the American Academy of Pediatrics, nearly 6.2 million children have been infected with the coronavirus. In the past six weeks, the number of infections has exceeded 1.1 million following the surge in delta mutants.
Moderna is also working on a Covid-19 vaccine for primary school-age children. Pfizer and Moderna are also studying younger children until they are 6 months old. Results are expected later this year.
[ad_2]
Source link